-
PDF
- Split View
-
Views
-
Cite
Cite
S. G. De Hert, P. W. ten Broecke, E. Mertens, I. E. Rodrigus, B. A. Stockman, Effects of phosphodiesterase III inhibition on length‐dependent regulation of myocardial function in coronary surgery patients, BJA: British Journal of Anaesthesia, Volume 88, Issue 6, 1 June 2002, Pages 779–784, https://doi.org/10.1093/bja/88.6.779
Close - Share Icon Share
Abstract
Background. Phosphodiesterase III inhibitors increase myocardial contractility and decrease left ventricular (LV) afterload. We studied whether these effects altered LV response to an increase in cardiac load and affected length‐dependent regulation of myocardial function.
Methods. Before the start of cardiopulmonary bypass, a high‐fidelity pressure catheter was positioned in the left ventricle and the left atrium in 10 coronary surgery patients. LV response to an increased cardiac load, caused by leg elevation, was assessed during baseline conditions and after administration of milrinone at a dose of 20 µg kg–1 over 15 min. Effects on contraction were measured by changes in maximal rate of pressure development (dP/dtmax). Effects on relaxation were assessed by analysis of changes in maximum rate of pressure decrease and by analysis of the load dependency of myocardial relaxation (R = slope of the relation between the time constant of isovolumic relaxation and end systolic pressure).
Results. Milrinone increased dP/dtmax but measures of relaxation were unaltered. Leg elevation had more effect on measures of contraction and relaxation after milrinone than at baseline. The relationship between R and changes in dP/dtmax shifted downwards and to the right with milrinone, whereas the relationship between R and changes in end diastolic pressure (EDP) shifted downwards and to the left.
Conclusions. This suggests that milrinone improved contraction, reduced the load dependency of LV pressure decrease, and reduced the change in EDP after leg elevation.
Br J Anaesth 2002: 88: 779–84
Accepted for publication: January 15, 2002
Length‐dependent regulation of myocardial function is the ability of the heart to improve its performance when preload is increased. In the non‐failing heart, an increase in diastolic volume increases cardiac function12 but when a failing left ventricle is subjected to an additional load, it appears to be unable to recruit the Frank–Starling mechanism, and does not improve its function.34 In coronary surgery patients with an ejection fraction >40%, an increase in cardiac load by leg elevation caused a variable left ventricular (LV) response. Some patients showed improvement, whereas other patients showed either no change or even reduced LV function. This latter response was related to a deficient length‐dependent regulation of myocardial function, with a decrease in stroke volume and maximal rate of pressure development (dP/dtmax), delayed myocardial relaxation with enhanced load dependence of LV pressure (LVP) decline, and a more pronounced increase in end diastolic pressure (EDP).56 Therefore even in patients with reasonable baseline cardiac function, length‐dependent regulation of myocardial function may be impaired.
Milrinone inhibits phosphodiesterase III (PDE III) and this induces breakdown of cyclic adenosine monophosphate (cAMP), leading to increased myocardial contractility and vasodilation of vascular smooth muscle.78 The effects of milrinone on cardiac function therefore result from improved contractility and decreased afterload.
On the basis of these effects, we proposed that milrinone would not only improve baseline LV function but would also affect the length‐dependent regulation of myocardial function. We therefore compared the effects of leg elevation before and during administration of milrinone.
Methods
We studied 10 patients scheduled for elective coronary bypass surgery. The study was approved by the Institutional Ethical Committee (University Hospital Antwerp, Edegem, Belgium) and written informed consent was obtained. We excluded patients with an ejection fraction <40% because of the potential adverse haemodynamic effects of transducer insertion in these patients. We also excluded patients having repeat coronary surgery, concurrent valve repair or aneurysm resection, and patients with unstable angina or with valve disease.
All cardiac medication, with the exception of angiotensin converting enzyme inhibitors, was continued until the morning of surgery. Patients received routine monitoring with five‐lead ECG, radial and pulmonary artery catheters, pulse oximetry, capnography, and blood and urinary bladder temperature monitoring. Anaesthesia was induced with remifentanil 0.5 µg kg–1, diazepam 0.1 mg kg–1, and pancuronium bromide 0.1 mg kg–1, followed by remifentanil 0.4 µg kg–1 min–1 and sevoflurane 0.5–1% in an oxygen‐enriched air mixture (FiO2 0.5). Standard median sternotomy and pericardiotomy were performed and the aortic cannula was secured in place.
Experimental preparation
In each patient, two sterilized, pre‐zeroed catheter‐tip pressure transducers (MTCP3Fc catheter, Dräger Medical Electronics, Best, The Netherlands; frequency response 100 kHz) were inserted. One catheter was positioned in the left atrium and the other in the LV cavity, both via the right superior pulmonary vein. The catheters were connected to a Hewlett Packard monitor (HP78342A, Hewlett Packard, Brussels, Belgium). The output signals of the pressure transducer system were digitally recorded, together with the ECG signals, at 1 ms intervals (Codas, DataQ, Akron, OH). Zero and gain settings of the transducers were also checked against a high‐fidelity pressure gauge (Druck Ltd, Leicester, UK) after removal.
Experimental protocol
Heart rate was kept constant at a rate of 90 beats min–1 by atrioventricular sequential pacing. LV EDP was kept constant to ensure stable filling pressures throughout by slow administration of the priming fluid through the aortic cannula whenever necessary. Concentrations of the anaesthetic agents were not altered during the study. All measurements were obtained with ventilation stopped at end expiration. The measurements consisted of recordings of consecutive ECG and LVP tracings when systolic and diastolic pressures were increased by raising the lower end part of the surgical table by 45°, raising the legs, resulting in a rapid beat‐to‐beat increase in LVP.
After recording measurements in baseline conditions (baseline 1), the patients were given a continuous infusion of milrinone 20 µg kg–1 over 15 min. This dose has inotropic effects similar to the usual dose of 50 µg kg–1 but causes less hypotension.9 After a second set of measurements, milrinone was discontinued. When haemodynamic values had returned to baseline, the effects of leg elevation were measured again (baseline 2) to assess a possible time effect.
Data analysis
EDP was measured at the peak of the R wave on the ECG. The effects of leg elevation in the different conditions on LV load and function were evaluated by the changes in EDP, peak LVP, LVP at maximum rate of pressure decrease (dP/dtmin), end systolic pressure (ESP), and maximal rate of pressure development (dP/dtmax). The effects of leg elevation on the rate of LVP decline were measured by dP/dtmin and the time constant τ of isovolumic relaxation. τ was calculated using a monoexponential model with non‐zero asymptote using LVP values from dP/dtmin to mitral valve opening. The following equation was used: ln Pt=ln P0–time/τ. Time constant τ was linearly fitted to the corresponding ESP, and the slope R (ms per mm Hg) of this relation was calculated. R indicates changes in τ, induced by the changes in ESP, and expresses the afterload dependence of the rate of LVP decline.10 At least 10 consecutive beats were measured for the calculation of R.
Statistical analysis
Data before and after leg elevation in the different conditions were compared using a two‐way analysis of variance for repeated measurements. Interaction analysis revealed whether effects of leg elevation were different with milrinone. The Bonferroni–Dunn test was used to analyse differences between the different experimental conditions. Relationships between haemodynamic parameters were analysed using linear regression analysis computing Pearson’s correlation coefficient. Slopes and intercepts of the different relationships were compared using a t test. All P values were two‐tailed and P<0.05 was considered significant. Data are expressed as mean (sd).
Results
Table 1 summarizes the patient characteristics. None of the patients developed myocardial ischaemia or haemodynamic instability during the study.
Table 2 shows the effect of milrinone on LV and left atrial pressure in the 10 patients. Mean peak LVP and dP/dtmax increased, whereas the other values remained unchanged.
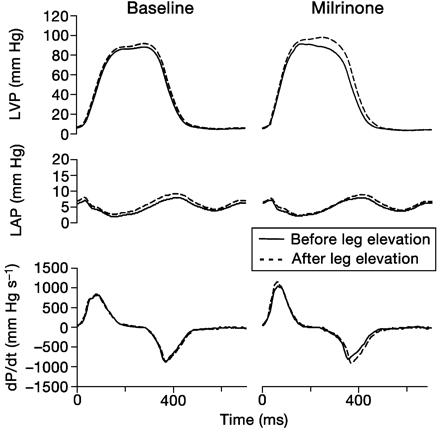
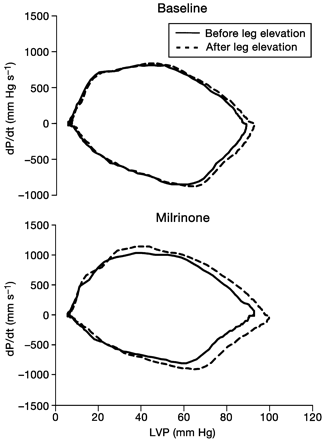
Figure 1 illustrates the effect of leg elevation before and during milrinone infusion in an individual patient. The effect of leg elevation on LVP was more pronounced during milrinone infusion. The effect of leg elevation on rate and pattern of pressure decline is illustrated with dP/dt vs pressure phase–plane plots (Fig. 2). On such a plot the cardiac cycle is read clockwise from the left, pressure increase being displayed above the zero line and pressure decrease below the zero line. In each panel of Figure 2, a beat after leg elevation is compared with the tracings obtained before leg elevation. Before milrinone, leg elevation increased peak LVP but rate and pattern of pressure increase and pressure decline remained unchanged. During milrinone administration, not only was the increase in LVP with leg elevation more pronounced but rate of pressure development and pressure decline was increased.
The effects of leg elevation in baseline conditions and during milrinone are summarized in Table 2. The increase in LVP, dP/dtmax and time from end diastole to dP/dtmin with leg elevation were significantly greater with milrinone compared with baseline. The increase in dP/dtmin with leg elevation was also more pronounced. τ increased with leg elevation in baseline conditions but remained unchanged during milrinone. The increase in EDP with leg elevation was less pronounced with milrinone. Sample correlation coefficients of the ESP–τ relationship yielded values of r>0.92 in all patients. The slope of this relationship quantified load dependence of relaxation. Compared with baseline, milrinone decreased R.
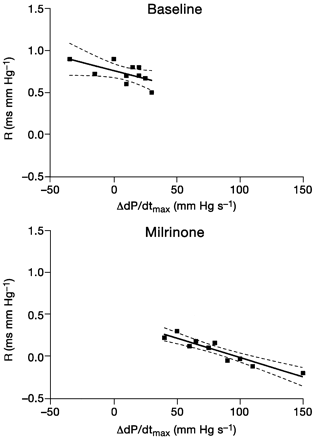
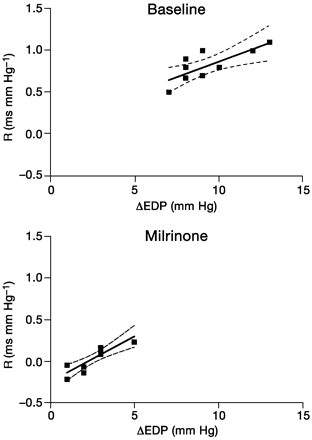
During the alteration in cardiac load by leg elevation, changes in measures of contraction and relaxation were coupled. Figure 3 illustrates the relationship between the afterload dependence of relaxation of LVP decline (R) and individual changes in dP/dtmax with leg elevation at baseline conditions and with milrinone. At baseline, R and changes in dP/dtmax were related according to the equation: y=0.68–0.004x (r=0.75; P<0.05). With milrinone, the relationship between R and changes in dP/dtmax was similar (y=0.59–0.005x; r=0.89; P<0.05) but the data demonstrated a shift downwards and to the right compared with the data in baseline conditions. Figure 4 illustrates the relationship between R and individual changes in EDP with leg elevation at baseline conditions and with milrinone. At baseline, R and changes in EDP were related according to the equation: y=–0.13+0.05x (r=0.72; P<0.05). With milrinone, the relationship between R and changes in EDP was also similar (y=–0.23 0.10x; r=0.77; P<0.05) but the data were shifted downwards and to the left compared with the data in baseline conditions.
Data in baseline 1 and 2 were similar (Table 2), excluding a possible time effect in the present observations.
Discussion
In these patients about to have coronary artery surgery and with a preoperative ejection fraction >40%, milrinone not only improved baseline myocardial function but also improved the length‐dependent regulation of myocardial function. Length‐dependent regulation of myocardial function is the ability of the heart to improve its performance when preload is increased. This is characterized by an increase in dP/dtmax and a decreased load dependence of myocardial relaxation when cardiac loading is increased with leg elevation.
Milrinone improves baseline cardiac function by increasing contractility and reducing afterload. The inotropic and vasodilating activities of milrinone are caused by a dose‐dependent inhibition of PDE III with an increase in intracellular cAMP.78 The mechanisms involved in the improvement of the length‐dependent regulation of myocardial function with milrinone are less clear. A possible explanation for this observation is related to the physiological concept of relative load.11–13 This concept was developed from experimental observations in isolated cardiac muscle and in animal experiments in vivo.14 Relative load is defined as the ratio of baseline systolic LVP to isovolumic systolic pressure and is expressed as a percentage. A low relative load (<70%) is associated with normal cardiac function, whereas a high relative load (>80%) suggests cardiac dysfunction. In this case, afterload reserve is limited. Afterload reserve is the capacity of the ventricle to respond to an afterload elevation with a limited increase in systolic volume and no slowing of LVP decline11–13 The afterload mismatch at high relative load slows LVP decline and shifts the diastolic pressure–volume relationship upwards, with increased EDP and diastolic failure.1315
Assessment of relative load cannot be obtained clinically by calculating the ratio of actual systolic LVP to isovolumic systolic pressure. However, because of the close relationship between rate of LVP decline and cardiac loading conditions,10–14 the response of the rate of LVP decline to an increase in cardiac load could indicate if the ventricle is working at low or high relative load. If LV function improves and the rate of LVP decline accelerates when load is increased, this indicates that the relative load at which the ventricle is working is low. Conversely, when LV function decreases and the rate of LVP decline is slower, this indicates that the ventricle is working at high relative load. Previous observations in coronary surgery patients showed that an increase in cardiac load caused a variable haemodynamic response. Some patients showed improvement, whereas other patients showed either no change or even impairment of LV function. These patients developed a decrease in dP/dtmax, and a delayed myocardial relaxation with enhanced load dependence of LVP decline.561617 This response corresponds to what can be expected of a ventricle working at high relative load. The response of the left ventricle to an increase in cardiac load with leg elevation is modulated by changes in cardiac contractility and cardiac loading conditions.18–20
Milrinone increases myocardial contractility and reduces ventricular afterload,78 thus reducing the relative load at which the ventricle is working. By these mechanisms afterload reserve should increase. LVP decline is accelerated, with a concomitant decrease in the changes in EDP caused by leg elevation (Fig. 4). This shifts the diastolic pressure–volume relationship to the left, to a better position on the Frank–Starling curve, with improvement of the length‐dependent regulation of myocardial function.
Several aspects of the methods deserve attention. We studied patients with reasonable baseline cardiac function (preoperative ejection fraction >40%). Further studies are needed of milrinone in patients with severely impaired baseline LV function (preoperative ejection fraction <30%). Heart rate during the protocol was regulated with cardiac pacing, to exclude variations in heart rate between patients and within the same patient as a confounding factor. However, pacing alters the normal LV conduction patterns. This could enhance the load dependency of LVP decline, as is seen experimentally in dogs.21 The current data were obtained with an open chest and an open pericardium. After pericardiotomy, the EDP–dimension relationship moves to the right,1722 with improvement of the Frank–Starling mechanism compared with closed‐chest conditions.17 The present results should not be applied to closed‐chest conditions without further study. We studied anaesthetized patients. Neurohumoral reflexes, including those mediating cardiac function, may have been blunted or altered by anaesthesia. In addition, all patients were taking long‐term preoperative oral β‐blocking medication. This could have influenced the current observations because of the effects of β‐blocking medication on rate of LVP decline.2324
In conclusion, in our study of coronary surgery patients, milrinone not only improved baseline LV function but also improved the length‐dependent regulation of myocardial function, suggesting that phosphodiesterase III inhibitors improve the Frank–Starling mechanism.
Fig 1 Representative example of the effects of leg elevation in baseline conditions and after milrinone. In the presence of milrinone, the effect of leg elevation on LV haemodynamics is more pronounced.
Fig 2 dP/dt vs pressure phase–plane plots illustrating the effects of leg elevation on rate and pattern of pressure decline. In each panel, a beat after leg elevation is compared with the baseline beat. With milrinone, the effect of leg elevation was more pronounced.
Fig 3 Illustration of the relation between afterload dependence of relaxation of LV pressure decline (R) and individual changes in dP/dtmax with leg elevation at baseline conditions and with milrinone.
Fig 4 Illustration of the relation between afterload dependence of relaxation of LV pressure decline (R) and individual changes in EDP with leg elevation at baseline conditions and with milrinone.
Patient characteristics. Data are mean (sd or range)
| Male/female ratio | 8/2 |
| Age (yr) | 67 (58–74) |
| Height (cm) | 171 (10) |
| weight (kg) | 79 (11) |
| Diabetes | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Previous acute myocardial infarction | 3 |
| Hypertension | 10 |
| Ejection fraction (%) | 53 (7) |
| Medication | |
| β‐blocking agents | 10 |
| Angiotensin‐converting enzyme inhibitors | 3 |
| Calcium channel blockers | 5 |
| Nitrates | 6 |
| Male/female ratio | 8/2 |
| Age (yr) | 67 (58–74) |
| Height (cm) | 171 (10) |
| weight (kg) | 79 (11) |
| Diabetes | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Previous acute myocardial infarction | 3 |
| Hypertension | 10 |
| Ejection fraction (%) | 53 (7) |
| Medication | |
| β‐blocking agents | 10 |
| Angiotensin‐converting enzyme inhibitors | 3 |
| Calcium channel blockers | 5 |
| Nitrates | 6 |
Patient characteristics. Data are mean (sd or range)
| Male/female ratio | 8/2 |
| Age (yr) | 67 (58–74) |
| Height (cm) | 171 (10) |
| weight (kg) | 79 (11) |
| Diabetes | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Previous acute myocardial infarction | 3 |
| Hypertension | 10 |
| Ejection fraction (%) | 53 (7) |
| Medication | |
| β‐blocking agents | 10 |
| Angiotensin‐converting enzyme inhibitors | 3 |
| Calcium channel blockers | 5 |
| Nitrates | 6 |
| Male/female ratio | 8/2 |
| Age (yr) | 67 (58–74) |
| Height (cm) | 171 (10) |
| weight (kg) | 79 (11) |
| Diabetes | 2 |
| Chronic obstructive pulmonary disease | 1 |
| Previous acute myocardial infarction | 3 |
| Hypertension | 10 |
| Ejection fraction (%) | 53 (7) |
| Medication | |
| β‐blocking agents | 10 |
| Angiotensin‐converting enzyme inhibitors | 3 |
| Calcium channel blockers | 5 |
| Nitrates | 6 |
Left ventricular and left atrial haemodynamic data before (baseline 1), during and after (baseline 2) milrinone. Data are mean (sd). EDP, end diastolic pressure; LVP, left ventricular pressure; ESP, end systolic pressure; R, afterload dependence of myocardial relaxation; Δ, effect of leg elevation on variable; *P<0.05 vs baseline
| Baseline 1 | Milrinone | Baseline 2 | |
| Peak A‐wave (mm Hg) | 12 (3) | 13 (3) | 12 (2) |
| Peak V‐wave (mm Hg) | 17 (4) | 17 (4) | 16 (2) |
| EDP (mm Hg) | 6 (1) | 6 (2) | 6 (1) |
| Peak LVP (mm Hg) | 89 (6) | 101 (5)* | 90 (5) |
| ESP (mm Hg) | 56 (7) | 54 (9) | 57 (7) |
| dP/dtmax (mm Hg s–1) | 856 (96) | 1036 (99)* | 846 (79) |
| dP/dtmin (mm Hg s–1) | 704 (79) | 715 (118) | 699 (98) |
| τ (ms) | 60 (8) | 61 (6) | 61 (6) |
| Δ peak A‐wave (mm Hg) | 5 (1) | 4 (1) | 4 (2) |
| Δ peak V‐wave (mm Hg) | 6 (2) | 6 (3) | 6 (2) |
| Δ EDP (mm Hg) | 8 (2) | 4 (1)* | 7 (1) |
| Δ peak LVP (mm Hg) | 12 (4) | 18 (3)* | 12 (5) |
| Δ ESP (mm Hg) | 6 (3) | 7 (4) | 6 (4) |
| Δ dP/dtmax (mm Hg s–1) | 13 (30) | 85 (39)* | 20 (29) |
| Δ dP/dtmin (mm Hg s–1) | 162 (75) | 296 (129)* | 149 (58) |
| Δ τ (ms) | 4 (1) | 0 (2)* | 4 (2) |
| R (ms per mm Hg) | 0.65 (0.23) | 0.08 (0.16)* | 0.58 (0.11) |
| Baseline 1 | Milrinone | Baseline 2 | |
| Peak A‐wave (mm Hg) | 12 (3) | 13 (3) | 12 (2) |
| Peak V‐wave (mm Hg) | 17 (4) | 17 (4) | 16 (2) |
| EDP (mm Hg) | 6 (1) | 6 (2) | 6 (1) |
| Peak LVP (mm Hg) | 89 (6) | 101 (5)* | 90 (5) |
| ESP (mm Hg) | 56 (7) | 54 (9) | 57 (7) |
| dP/dtmax (mm Hg s–1) | 856 (96) | 1036 (99)* | 846 (79) |
| dP/dtmin (mm Hg s–1) | 704 (79) | 715 (118) | 699 (98) |
| τ (ms) | 60 (8) | 61 (6) | 61 (6) |
| Δ peak A‐wave (mm Hg) | 5 (1) | 4 (1) | 4 (2) |
| Δ peak V‐wave (mm Hg) | 6 (2) | 6 (3) | 6 (2) |
| Δ EDP (mm Hg) | 8 (2) | 4 (1)* | 7 (1) |
| Δ peak LVP (mm Hg) | 12 (4) | 18 (3)* | 12 (5) |
| Δ ESP (mm Hg) | 6 (3) | 7 (4) | 6 (4) |
| Δ dP/dtmax (mm Hg s–1) | 13 (30) | 85 (39)* | 20 (29) |
| Δ dP/dtmin (mm Hg s–1) | 162 (75) | 296 (129)* | 149 (58) |
| Δ τ (ms) | 4 (1) | 0 (2)* | 4 (2) |
| R (ms per mm Hg) | 0.65 (0.23) | 0.08 (0.16)* | 0.58 (0.11) |
Left ventricular and left atrial haemodynamic data before (baseline 1), during and after (baseline 2) milrinone. Data are mean (sd). EDP, end diastolic pressure; LVP, left ventricular pressure; ESP, end systolic pressure; R, afterload dependence of myocardial relaxation; Δ, effect of leg elevation on variable; *P<0.05 vs baseline
| Baseline 1 | Milrinone | Baseline 2 | |
| Peak A‐wave (mm Hg) | 12 (3) | 13 (3) | 12 (2) |
| Peak V‐wave (mm Hg) | 17 (4) | 17 (4) | 16 (2) |
| EDP (mm Hg) | 6 (1) | 6 (2) | 6 (1) |
| Peak LVP (mm Hg) | 89 (6) | 101 (5)* | 90 (5) |
| ESP (mm Hg) | 56 (7) | 54 (9) | 57 (7) |
| dP/dtmax (mm Hg s–1) | 856 (96) | 1036 (99)* | 846 (79) |
| dP/dtmin (mm Hg s–1) | 704 (79) | 715 (118) | 699 (98) |
| τ (ms) | 60 (8) | 61 (6) | 61 (6) |
| Δ peak A‐wave (mm Hg) | 5 (1) | 4 (1) | 4 (2) |
| Δ peak V‐wave (mm Hg) | 6 (2) | 6 (3) | 6 (2) |
| Δ EDP (mm Hg) | 8 (2) | 4 (1)* | 7 (1) |
| Δ peak LVP (mm Hg) | 12 (4) | 18 (3)* | 12 (5) |
| Δ ESP (mm Hg) | 6 (3) | 7 (4) | 6 (4) |
| Δ dP/dtmax (mm Hg s–1) | 13 (30) | 85 (39)* | 20 (29) |
| Δ dP/dtmin (mm Hg s–1) | 162 (75) | 296 (129)* | 149 (58) |
| Δ τ (ms) | 4 (1) | 0 (2)* | 4 (2) |
| R (ms per mm Hg) | 0.65 (0.23) | 0.08 (0.16)* | 0.58 (0.11) |
| Baseline 1 | Milrinone | Baseline 2 | |
| Peak A‐wave (mm Hg) | 12 (3) | 13 (3) | 12 (2) |
| Peak V‐wave (mm Hg) | 17 (4) | 17 (4) | 16 (2) |
| EDP (mm Hg) | 6 (1) | 6 (2) | 6 (1) |
| Peak LVP (mm Hg) | 89 (6) | 101 (5)* | 90 (5) |
| ESP (mm Hg) | 56 (7) | 54 (9) | 57 (7) |
| dP/dtmax (mm Hg s–1) | 856 (96) | 1036 (99)* | 846 (79) |
| dP/dtmin (mm Hg s–1) | 704 (79) | 715 (118) | 699 (98) |
| τ (ms) | 60 (8) | 61 (6) | 61 (6) |
| Δ peak A‐wave (mm Hg) | 5 (1) | 4 (1) | 4 (2) |
| Δ peak V‐wave (mm Hg) | 6 (2) | 6 (3) | 6 (2) |
| Δ EDP (mm Hg) | 8 (2) | 4 (1)* | 7 (1) |
| Δ peak LVP (mm Hg) | 12 (4) | 18 (3)* | 12 (5) |
| Δ ESP (mm Hg) | 6 (3) | 7 (4) | 6 (4) |
| Δ dP/dtmax (mm Hg s–1) | 13 (30) | 85 (39)* | 20 (29) |
| Δ dP/dtmin (mm Hg s–1) | 162 (75) | 296 (129)* | 149 (58) |
| Δ τ (ms) | 4 (1) | 0 (2)* | 4 (2) |
| R (ms per mm Hg) | 0.65 (0.23) | 0.08 (0.16)* | 0.58 (0.11) |
References
Plotnick GD, Becker LC, Fisher ML, et al. Use of the Frank‐Starling mechanism during submaximal versus maximal upright exercise.
Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man.
Komamura K, Shannon RP, Ihara T, et al. Exhaustion of Frank‐Starling mechanism in conscious dogs with heart failure.
Schwinger RHG, Böhm M, Koch A, et al. The failing human heart is unable to use the Frank‐Starling mechanism.
De Hert SG, Gillebert TC, ten Broecke PW, Mertens E, Rodrigus IR, Moulijn AC. Contraction‐relaxation coupling and impaired left ventricular performance in coronary surgery patients.
De Hert SG, Gillebert TC, ten Broecke PW, Moulijn AC. Length‐dependent regulation of left ventricular function in coronary surgery patients.
Honerjäger P. Pharmacology of bipyridine phosphodiesterase III inhibitors.
Skoyles JR, Sherry KM. Pharmacology, mechanisms of action and uses of selective phosphodiesterase inhibitors.
De Hert SG, Moens MM, Jorens PG, Delrue GL, De Paep RJ, Vermeyen KM. Comparison of two different loading doses of milrinone for weaning from cardiopulmonary bypass. J Cardiothor Vasc Anesth
Eichhorn EJ, Willard JE, Alvarez L, et al. Are contraction and relaxation coupled in patients with and without congestive heart failure?
Gillebert TC, Leite‐Moreira AF, De Hert SG. Relaxation– systolic pressure relation: a load‐independent assessment of left ventricular contractility.
Gillebert TC, Leite‐Moreira AF, De Hert SG. The hemodynamic manifestation of normal myocardial relaxation. A framework for experimental and clinical evaluation.
Gillebert TC, Leite‐Moreira AF, De Hert SG. Load‐dependent diastolic dysfunction in heart failure.
Leite‐Moreira AF, Gillebert TC. Nonuniform course of left ventricular pressure fall and its regulation by load and contractile state.
Leite‐Moreira AF, Correia‐Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction.
De Hert SG, Van der Linden PJ, ten Broecke PW, De Mulder PA, Rodrigus IE, Adriaensen HF. Assessment of length‐dependent regulation of myocardial function in coronary surgery patients using transmitral flow velocity patterns.
De Hert SG, ten Broecke PW, Rodrigus IE, Mertens E, Stockman BA, Vermeyen KM. The effect of the pericardium on length‐dependent regulation of left ventricular function in coronary surgery patients.
De Hert SG, Van der Linden PJ, ten Broecke PW, et al. The effects of β‐adrenergic stimulation on the length‐dependent regulation of myocardial function in coronary surgery patients.
De Hert SG, Van der Linden PJ, ten Broecke PW, Sermeus LA, Gillebert TC. Effects of nicardipine and urapidil on length‐dependent regulation of myocardial function in coronary artery surgery patients.
De Hert SG, Van der Linden PJ, ten Broecke PW, Vermeylen KT, Rodrigus IE, Stockman BA. Effects of desflurane and sevoflurane on length‐dependent regulation of myocardial function in coronary surgery patients.
Lew WYW. Asynchrony and ryanodine modulate load‐dependent relaxation in the canine left ventricle.
Shirato K, Shabetai R, Bhargava V, Franklin D, Ross J Jr. Alteration of the left ventricular pressure‐segment length relation produced by the pericardium. Effects of cardiac distension and afterload reduction in conscious dogs.
Karliner JS, LeWinter MM, Mahler F, Engler R, O’Rourke RA. Pharmacologic and hemodynamic influences on the rate of isovolumic left ventricular relaxation in the normal conscious dog.
Author notes
Departments of 1Anaesthesiology and 2Cardiac Surgery, University Hospital Antwerp, Wilrijkstraat 10, B‐2650 Edegem, Belgium*Corresponding author